About Us
Skanray, with research and development centres in India and Europe, is a leading manufacturer of highly precise medical equipment in the healthcare sector with a dedicated team of 800+ employees, operations in 7 countries, and unique exports to 80 nations. The company’s in-house manufacturing capabilities support a broad portfolio of life-saving solutions. Skanray’s global reputation is built on its exceptional customer support, demonstrated by over 150,000 installations worldwide.
With over 80 patents, Skanray contributes to over 50 advanced medical devices, including diagnostic and critical care systems, all meeting rigorous standards like US FDA, UL, and CE. Its manufacturing facilities boast of ISO 9001:2015, ISO 14001:2015, ISO 13485:2016, and ISO 45001:2018, setting a high standard in the healthcare industry, and making Skanray a trusted global player.
2007 – Skanray, founded by 5 experienced, ex-GE, medical technology engineers.
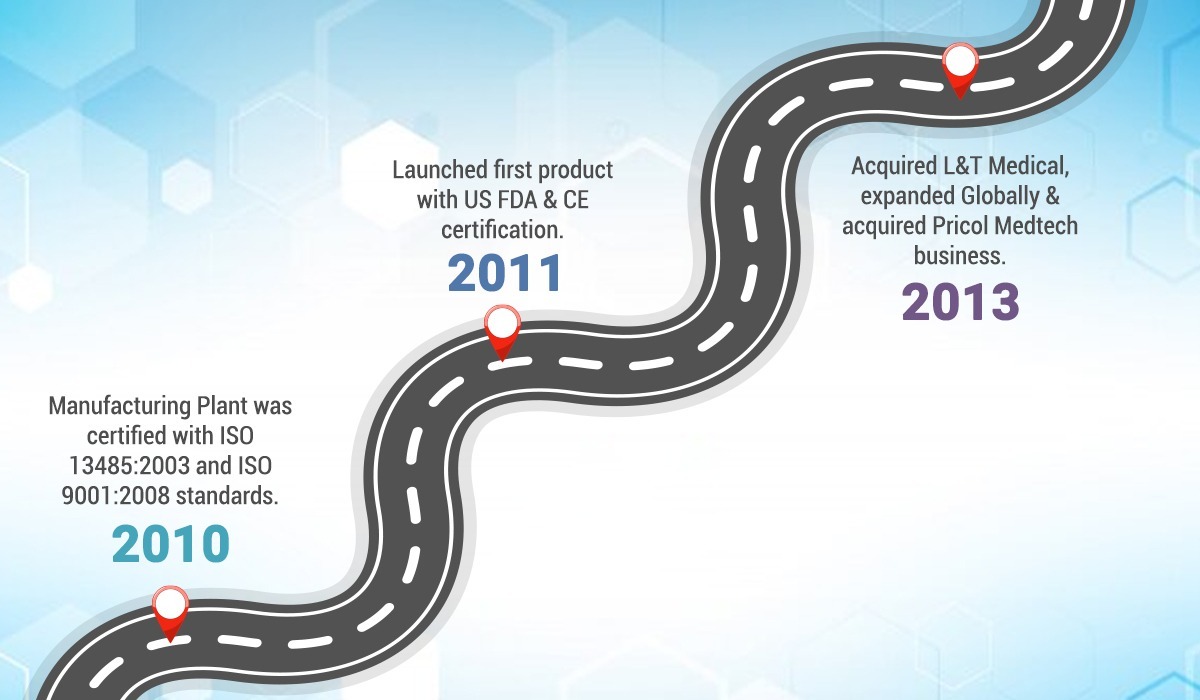
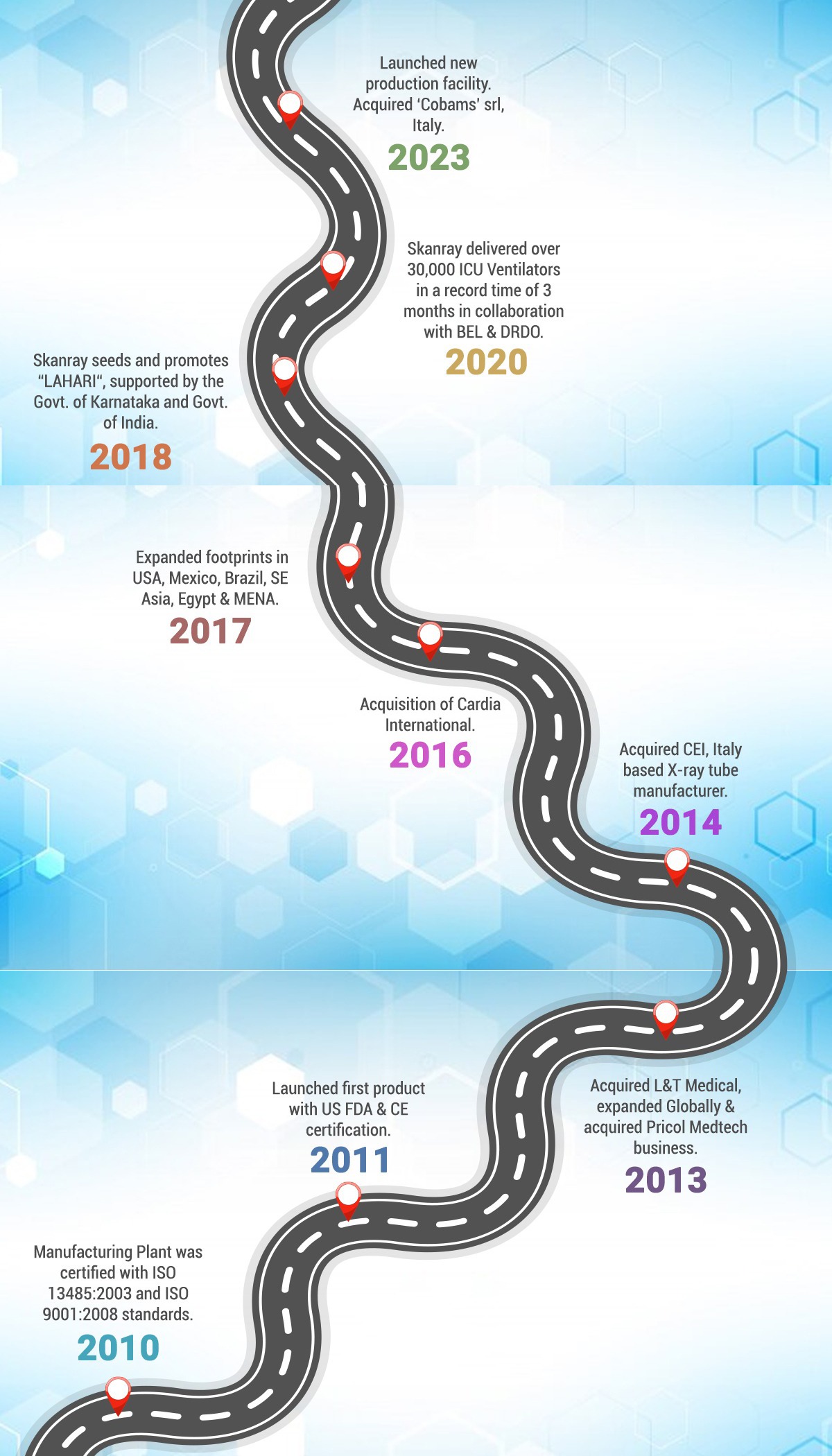
2010 – Manufacturing Plant was certified with ISO 13485:2003 and ISO 9001:2008 standards.
2011 – Launched first product Intraskan DC with US FDA & CE certification.
2012 – Launched mobile X-ray & critical care systems.
2013 – Acquired L&T Medical, expanded Globally & acquired Pricol Medtech business.
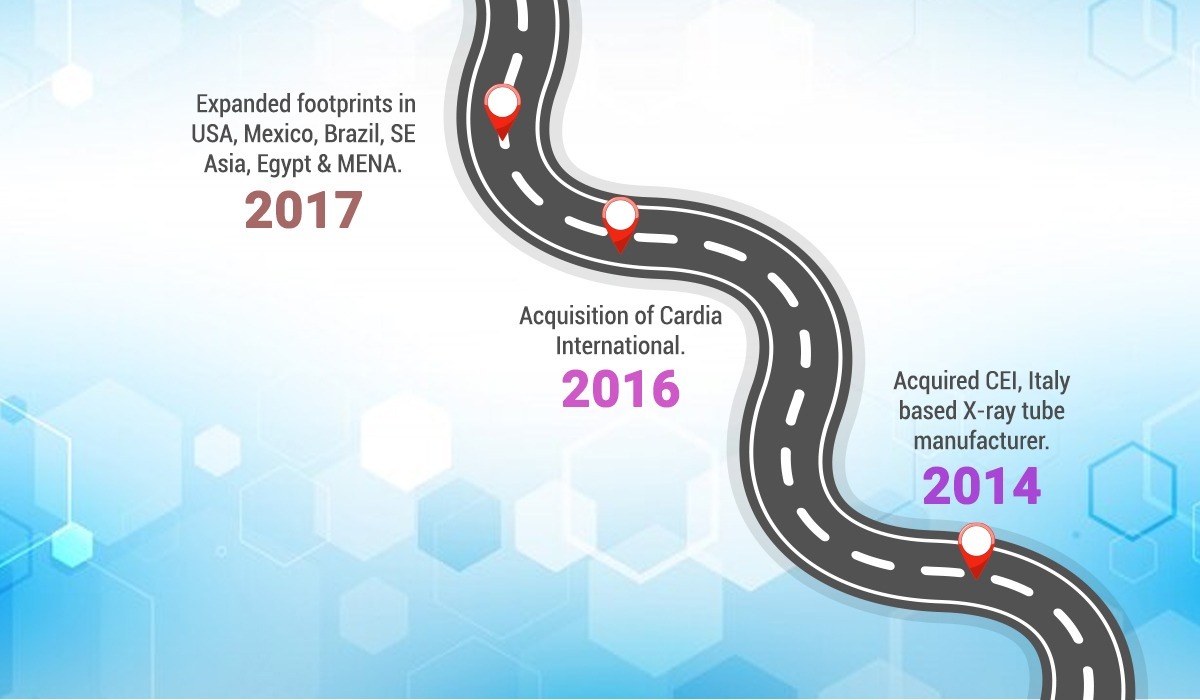
2014 – Commercial launch of Fixed X-ray products. Acquired CEI, Italy based X-ray tube manufacturer.
2016 – Acquisition of Cardia International. Commercial launch of Skanray’s first Veterinary product.
2017 – Expanded footprints in USA, Mexico, Brazil, SE Asia, Egypt & MENA.
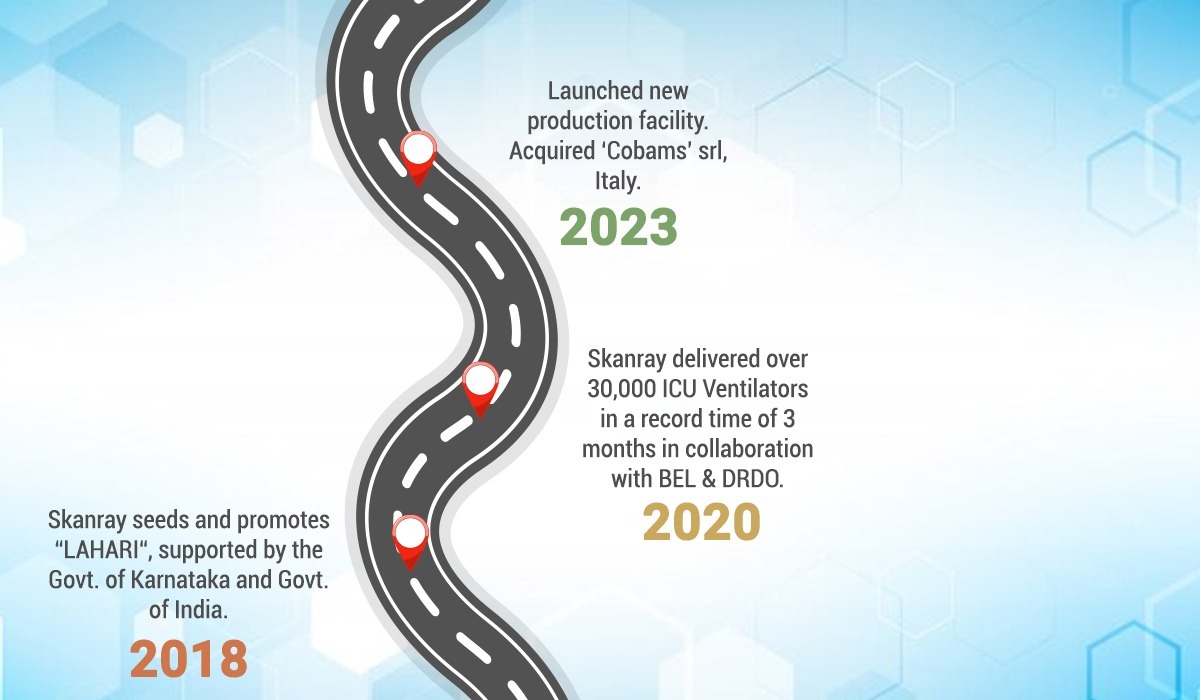
2018 – Skanray seeds and promotes “LAHARI“, supported by the Govt. of Karnataka and Govt. of India.
2020 – Skanray delivered over 30,000 ICU Ventilators in a record time of 3 months in collaboration with BEL & DRDO.
2023 – Launched new production facility. Acquired ‘Cobams’ srl, Italy.
Facilities
Corporate Office – R&D Centre
- 88,000 sq. ft. facility with a 70,000 sq. ft. state-of-the-art R&D infrastructure and manufacturing line for high voltage components
- Large global R&D team focused on developing modular technology platforms that can be used across multiple products
- Equipped with degassing vacuum ovens, oil filtration systems, lead chambers and shielded rooms for X-ray testing, environment simulation chambers, reliability testing apparatus, etc., to facilitate R&D and manufacturing of high voltage components
- High voltage and precision electronics assembly line
- Zero discharge environment-friendly facility


Operations Plant
- 2,50,000 sq. ft. with a 1,50,000 sq. ft. state-of-the-art manufacturing infrastructure
- ISO 13485 and ISO 9001 certified manufacturing facility
- Has an in-house EMI/EMC lab that ensures that our product designs exceed the requirements of IEC 61000-4 and IEC 60601-1
- Zero discharge environment-friendly facility
Skan-X Radiology Devices SpA, Italy
- Specialises in X-ray tubes for medical and industrial applications
- Partners with the Bologna University of Physics for advanced research; specialises in X-ray physics
- Manufacturing unit houses state-of-the-art infrastructure producing high-quality X-ray tubes with proprietary manufacturing processes
- Leads the European market with over 60% share
- Long-term suppliers to Siemens, Kodak-Trophy, Villa, Sirona-Bluex, and Palodex – some of the well-known brands in X-ray equipment

Lahari, Mysore
Lahari is one of the country’s first advanced electronic test facilities funded by the Ministry of Electronics and Information Technology, Government of India and Government of Karnataka, in partnership with the Indian electronics industry. Skanray is the core promoter of Lahari.
A world-class facility for providing various testing, quality & regulatory needs of the industry, Lahari is a ONE-STOP PRODUCT DEVELOPMENT & COMPLIANCE FACILITY dedicated to the electronics manufacturing sector.